Showing 25–36 of 64 resultsSorted by latest
Product Code: 4AWT
Washer Test
(0 Reviews)
- Data Sheet
- 4AWT
Product Code: 4ADXR15
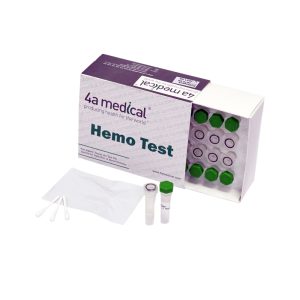
Hemo test
(0 Reviews)
Before the sterilization process of medical equipment and surgical instruments, there is a prewashing procedure. By the means of this procedure, blood residues remaining on especially surgical instruments that cause cross infection is surely cleaned. After Washing/Disinfection steps, there might be some residues or contaminants left in varying colors that can be observed. It is very essential to identify if the residue is of blood or corrosion, in order to carry out sterilization properly or otherwise to dispose the equipment. This blood residue test developed in our laboratories is an indicator which changes color only with a specific enzyme in the blood. By using this test, it is very easy to identify trace amounts of blood. Without a need of long incubation time, our test is able to identify up to~0.1micrograms of blood in residue, faster than any other test methods, that is as short as 10 seconds
Product Code: 4AC
Cannula Control
(0 Reviews)
- Data Sheet
- 4AC
Product Code: 4APRO
Protein Test
(0 Reviews)
- Data Sheet
- 4APRO
Product Code: 4ASC
Sonicontrol
(0 Reviews)
- Data Sheet
- 4ASC
Product Code: 4AIP
Incubator 37C/59C
(0 Reviews)
- Data Sheet
- 4AIP
Product Code: 4ASBI
Steam Biological Indicator
(0 Reviews)
- Data Sheet
- 4AEOBI
Product Code: 4AEOBI
EO Biological Indicator
(0 Reviews)
- Data Sheet
- 4AEOBI
Product Code: 4APBI
Plasma Biological Indicator
(0 Reviews)
- Data Sheet
- 4APBI